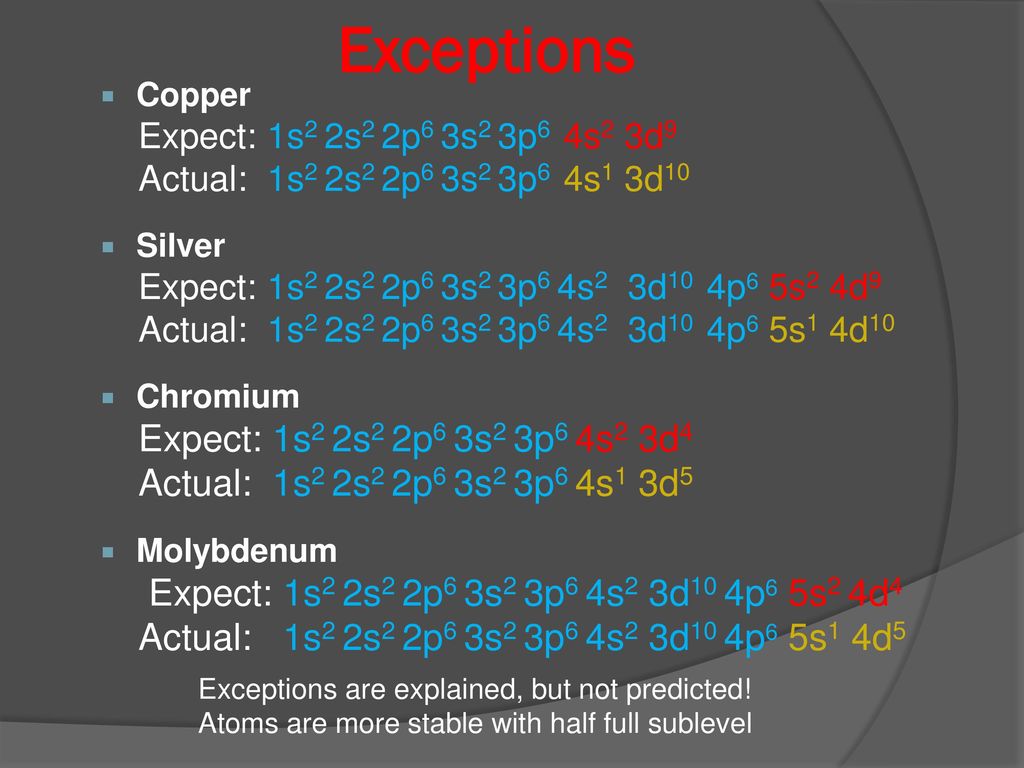
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 Element
Explanation 80 Hg I. Element 82 of Periodic table is Lead with atomic number 82 atomic weight 2072.

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 Youtube
Solve problems easily using our calculators and solvers.
. Lead is a post-transition metal element. Trivial name of Lead is tetrels crystallogens. La primera capa se compone de 2 electrones la segunda capa alberga 8 electrones la tercera capa está.
117 Téneso 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p5. Fuente de la imagen. 118 Oganesón 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6.
Get detailed information about chemistry and the elements. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 Or Xe. 1s22s22p63s23p63d104s24p64d104f145s25p65d106s2 1 S 0 term calculator.
Lead symbol Pb has a Face Centered Cubic structure and SlateGray color. El uranio tiene una configuración electrónica completa descrita como 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f3 6d1 7s2 su versión simplificada o abreviada es Rn 5f3 6d1 7s2. So we can look at all the atomic orbitals of the atoms of the XeF2 molecule.
1s2 2s2 2p5 Or F. Know everything about Lead Facts Physical Properties Chemical Properties Electronic configuration Atomic and Crystal Structure. Existen 92 electrones en total para el uranio la distribución de estos es.
Kr 4d10 5s2 5p6. Now we have to find out which AO combined with the. Ar 3d10 4s2 4p2 78994 1886 33 As ヒ素 Arsenic 749216 572 81 613 15 Ar 3d10 4s2 4p3 97886 古代 34 Se セレン Selenium 7896 479 217 685 16 Ar 3d10 4s2 4p4 97524 1817 35 Br 臭素 Bromine 79904 312 -7 59 17 Ar 3d10 4s2 4p5 118138 1826 36 Kr クリプトン Krypton 838 375 -157 -153 18 Ar 3d10 4s2 4p6 139996 1898 37 Rb.

Solved Which Of The Following Elements Has A Ground State Chegg Com

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2 5p6 Youtube
No comments for "1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 Element"
Post a Comment